The 2024 Medical Device Manufacturer Report
What’s keeping medical device manufacturers up at night? What are their top priorities and challenges? What new tactics are leading organizations employing to enhance efficiencies and reduce risks?
The 2024 Medical Device Manufacturing Report, based on a survey of 100 medical device manufacturing executives and engineers, has answers.
Preview Key Findings Below ⇓
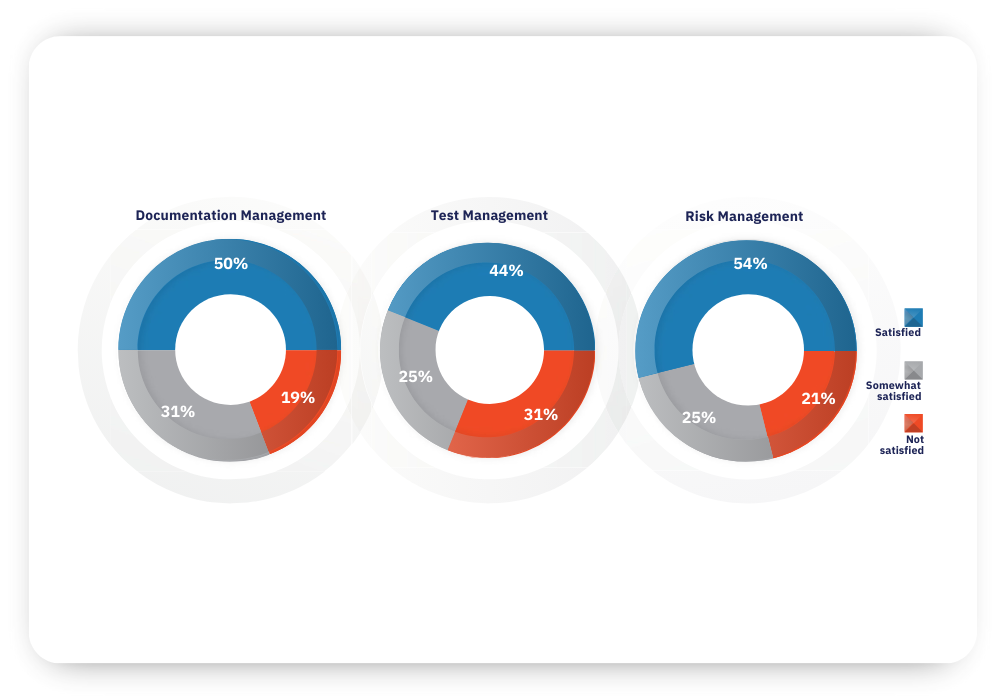
How satisfied are you with your organization’s current practices for:
Most medical device manufacturing executives and engineers indicated that their organization has less than optimal practices across critical areas: documentation management, test management, and risk management. In total, only about half of all survey respondents said they are very satisfied with their current practices within these areas.
How well do your current design control processes integrate across key domains?
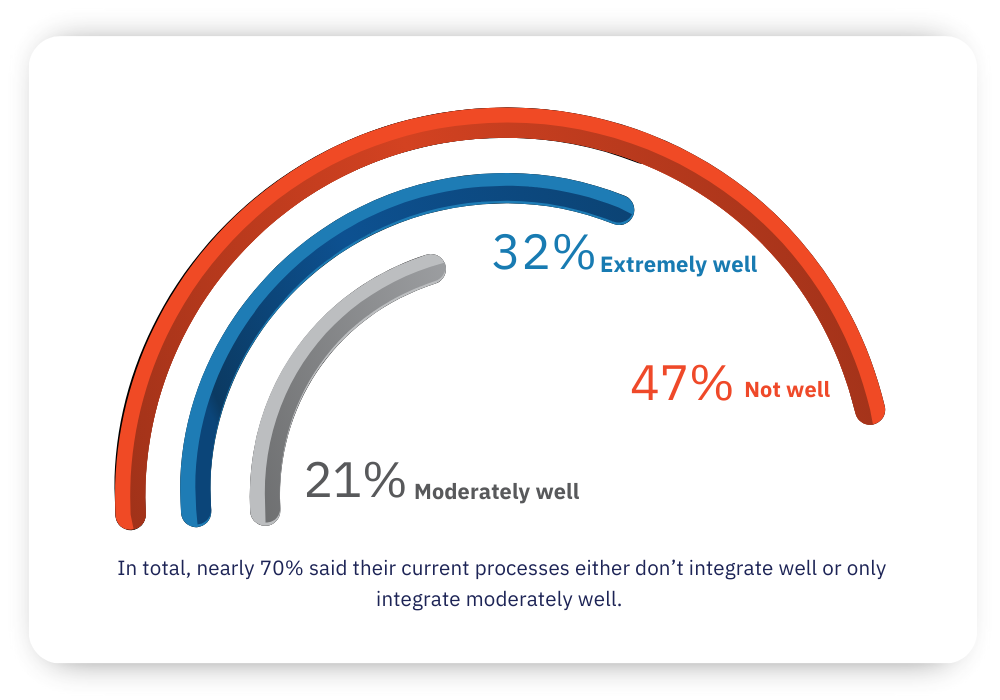
The findings also reveal that most medical device manufacturers struggle to integrate data and workflows across key domains (such as requirements, risk, and test management). Nearly 70% said their current processes either don’t integrate well or only integrate moderately well.
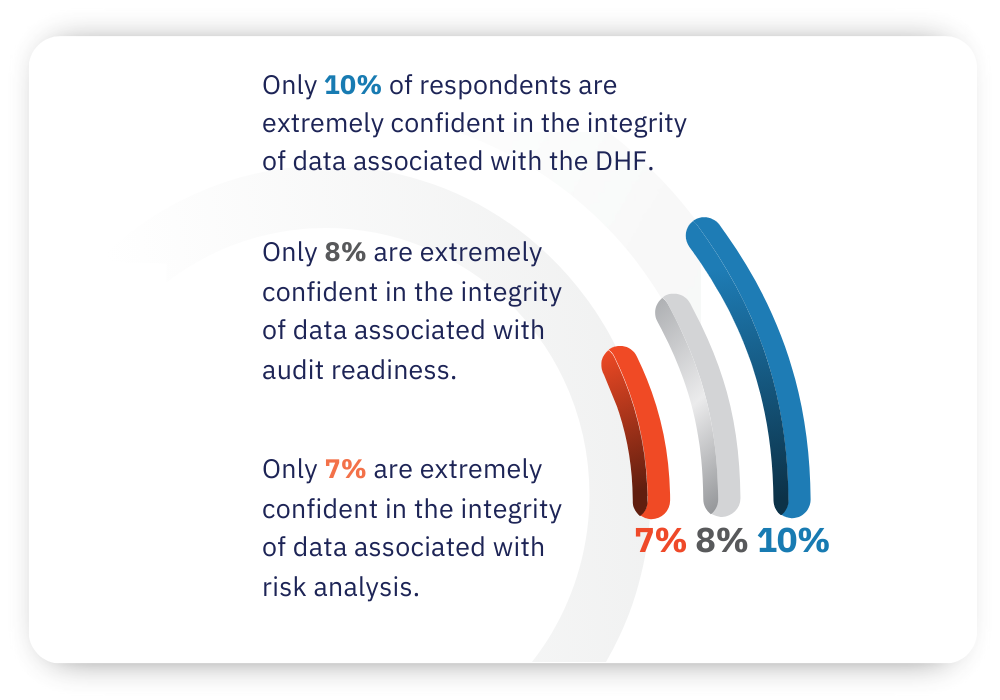
How confident are you in the integrity of your data?
For many medical device manufacturers, that lack of integration may be contributing to concerns related to the integrity of data. Overall, the survey found that: